Nanocomposite materials with ceramic particles used as modifying phases (e.g. TCP, SiO2, aluminosilicates) allow to obtain bioactive materials with the ability to stimulate tissue development, affect the cells development in a living organism and inhibit the formation of the bactericidal/bacteriostatic biofilm. That is why such materials are of high medical, veterinary or environmental interest. The biological advantages of nanocomposite materials can be anticipated by investigating the compatibility of fillers to both the matrix and the conditions in which they are to operate. An example of the deliberate use of nanometric silica is the determination of its potential to induce bioactivity in the bone tissue environment. Studies show that nanoparticles with higher surface development and the presence of hydroxyl groups induce the desired apatite nucleation as well as activate osteoblasts for faster proliferation. More importantly, such material properties favorably influence stem cells (MSC) as well as collagen secretion [1]. The results showed also that the bioactivity of nanocomposite materials can be anticipated by the zeta potential of nanoadditives. This can also be useful in the fabrication of nanocomposites with homogenous dispersion of nanofillers in a polymer matrix. Thorough characterization of nanofillers belonging to the same group of materials i.e., ceramic nanoparticles, may help to design the nanocomposite 3D scaffolds [2].
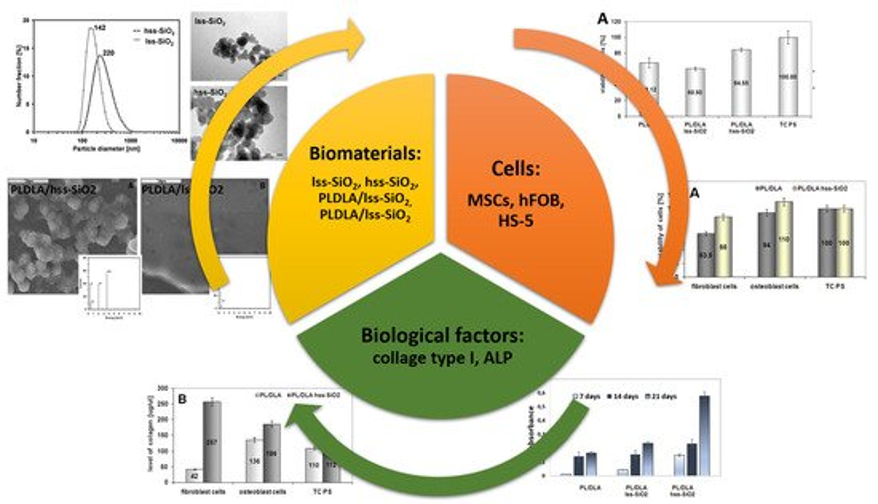
[1] Ł. Zych, A.M. Osyczka, A. Łącz, A. Różycka, W. Niemiec, A. Rapacz-Kmita, E. Dzierzkowska, E. Stodolak-Zych, How surface properties of silica nanoparticles influence structural, microstructural and biological properties of polymer nanocomposites, DOI: 10.3390/ma14040843
[2] E. Stodolak-Zych, R. Kurpanik, E. Dzierzkowska, M. Gajek, Ł. Zych, K. Gryń, A. Rapacz-Kmita Effects of montmorillonite and gentamicin addition on the properties of electrospun polycaprolactone fibers, DOI: 10.3390/ma14226905
When planning the use of ceramic nanoparticles in medical therapies, implants or nanocomposite layers, it is good to know the behavior of the particle in the presence of proteins and body fluids. It is known that the first stage of cell-material interaction involves phenomena related to surface wetting or protein adsorption. An example of such research is B4C particles, which can be successfully used as layers for biomechanical implants with enhanced tribological properties or particles used in oncology therapies. Studies indicate that the chemical state of B4C particles is the primary parameter to be checked before the material is tested in vitro/in vivo [3]. The proposed set of tests can be considered as a screening test to limit the number of materials for further in vitro testing.
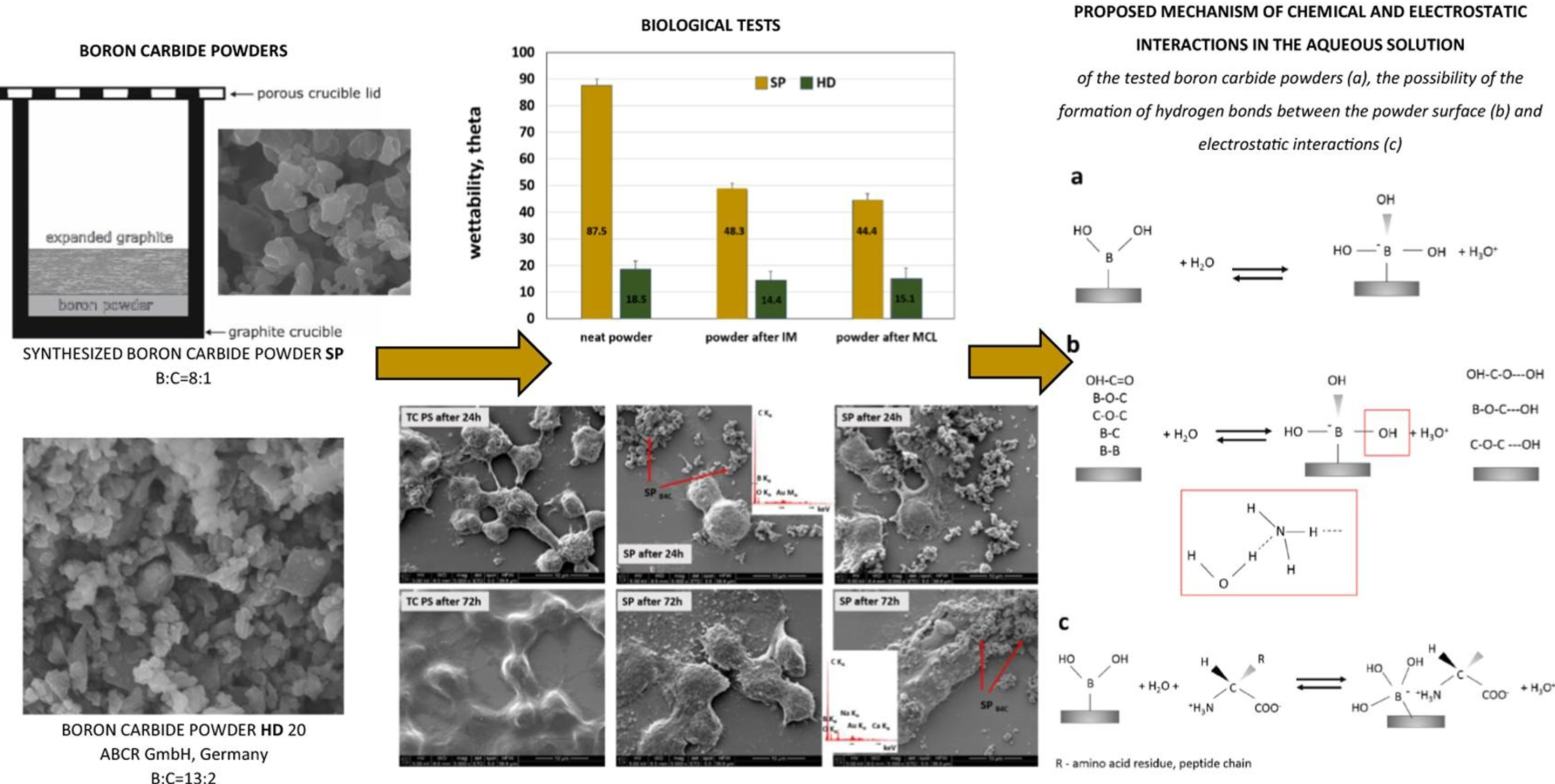
[3] E. Stodolak-Zych, A. Gubernat, A. Ścisłowska-Czarnecka, M. Chadzińska, Ł. Zych, D. Zientara, M. Nocuń, P. Jeleń, M. M. Bućko, The influence of surface chemical composition of particles of boron carbide powders on their biological properties, DOI: 10.1016/j.apsusc.2021.152380
Another example are polymer nanofibers modified with antibiotics and/or aseptic agents, the morphology of which (surface/volume porosity) ensures a controlled release of the pharmacological agent (Fig. 1). The appropriate fibrous membrane supporting the antibiotics makes it possible to control the drug release kinetics and concentration of the active agent. Thus, the material may be bactericidal for Gram positive bacteria and bacteriostatic for Gram negative bacteria [4]. Our research group focuses on creating fibrous substrates with a bactericidal and bacteriostatic effect which simultaneously enables the proper growth and development of somatic cells. Metric (nano)fibers with different morphology (porous, shiskebab) are excellent carriers of biologically active substances, e.g. antioxidants, drugs or aseptics [5].
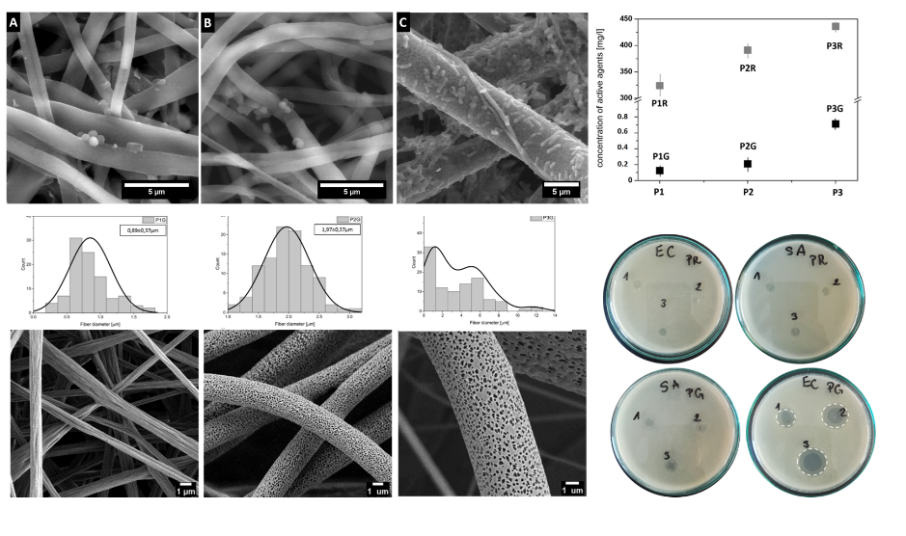
[4] E.Dzierzkowska, A. Ścisłowska-Czarnecka, D. Romaniszyn, S. Metwally, M. Chadzińska, E. Stodolak-Zych; Porous Poly(Lactic Acid) based fibres as drug carriers in active dressings, DOI: 10.37190/ABB-01548-2020-03
[5] ] E. Stodolak-Zych, E. Dzierzkowska, S. Metwally, M. Mikołajczyk, M. Gajek, A. Rapacz-Kmita; Multifunctional porous membranes with antibacterial properties, DOI: 10.1080/00914037.2018.1525719
Among various nanocomposite materials, layered silicates, such as montmorillonite (MMT), play a special role. The natural particles subjected to a directional modification may form a structure efficiently carrying active substances, e.g. antibiotics (gentamicin, kindamycin, biofuroxime). Such agents are released more easily from the polymer matrix of a defined porosity. Both the porosity value and the polymer type may determine the pace of the antibiotic release, prolonging its action in the diseased tissue.
Another bionanocomposite solution is the modification of biopolymer fibers (polysaccharide: chitosan/alginate) with active peptide fragments of collagen I or III in order to obtain a synthetic extracellular matrix (ECM). Polysaccharide-peptide systems are a biomimetic form intended to mimic the surroundings of natural tissue. The surface modification of biopolymer fibers is carried out by electrospraying methods and the volumetric modification - by chemical synthesis methods (addition) [6]. The biopolimer fibers confirmed their strong proliferative properties towards cartilage and bone cells in in vitro studies. The affinity of hydrogel fibrous systems, high water absorption and their surgical maneuverability are appreciated in veterinary medicine. The produced fibrous substrates are currently being tested in in vivo tests. Laboratory-synthesized peptides (their biologically active fragments, to be precise) are advantageous modifiers that influence the cellular response. This applies not only to fragments known and used by other researchers, such as RGD [7]. A novelty is the production of nanocomposite biolymer fibers modified with copper salts, which additionally reveal antibacterial properties. Thus, it is possible to speak of a substrate acting on two levels: stimulating cell adhesion (RGD) and inhibiting the action of bacteria (copper alginate).
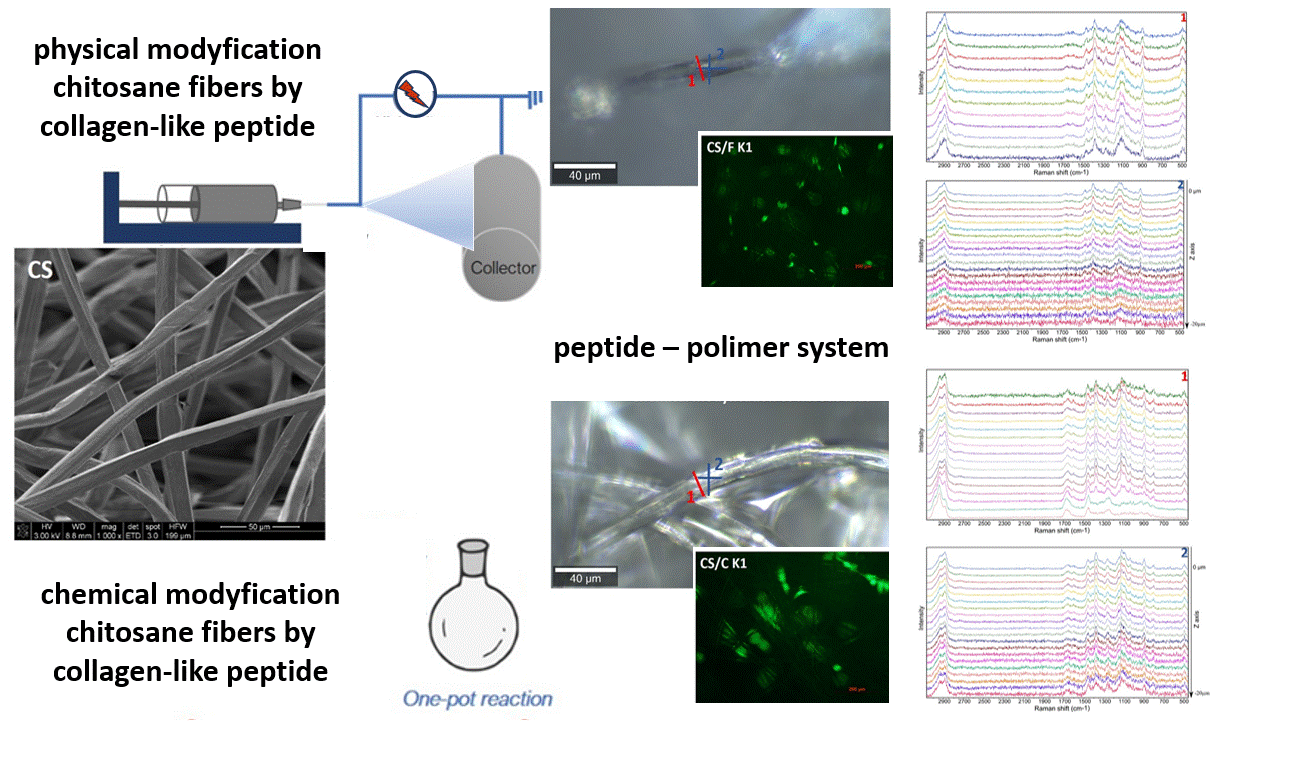
[6] E. Stodolak-Zych, P. Jeleń, E. Dzierzkowska, M. Krok-Borkowicz, Ł. Zych, M. Boguń, A. Rapacz-Kmita, B. Kolesińska; Modification of chitosan fibers with short peptides as a model of synthetic extracellular matrix, DOI: 10.1016/j.molstruc.2020.128061
[7] J. Fraczyk, [et al.], E. Stodolak-Zych, [et al.] Conjugates of copper alginate with arginine-glycine-aspartic acid (RGD) for potential use in regenerative medicine, DOI: 10.3390/ma13020337
Research on new materials for medical applications should complete with clinical trials conducted in accordance with GLP. Before this stage, the potential implant must be verified in preclinical studies on animals. The choice of the animal model as well as the argumentation of in vivo tests requires the consent of the Ethics Committee. Thanks to the cooperation with the Institute of Zootechnics PIB as well as with the university unit OMEI, we conduct in vivo research. Working in an interdisciplinary research team allows us to check a number of factors and their correlations with material behaviour in vitro. An example of such research are bone implants made of nanocomposite membrane, which are planned to be used in the reconstruction of the ruptured knee ligament (ACL) [8]. The most important parameter here is not only the return to full function of the animal (including clinical observations of the implant site) but also the quality of the bone tunnel-implant connection assessed in biomechanical tests. The final stage is always the histopathological evaluation of the tissues surrounding the implant. Only the set of these data allows an informed qualification of the implant for clinical trials with the participation of humans in hospitals selected for this task.
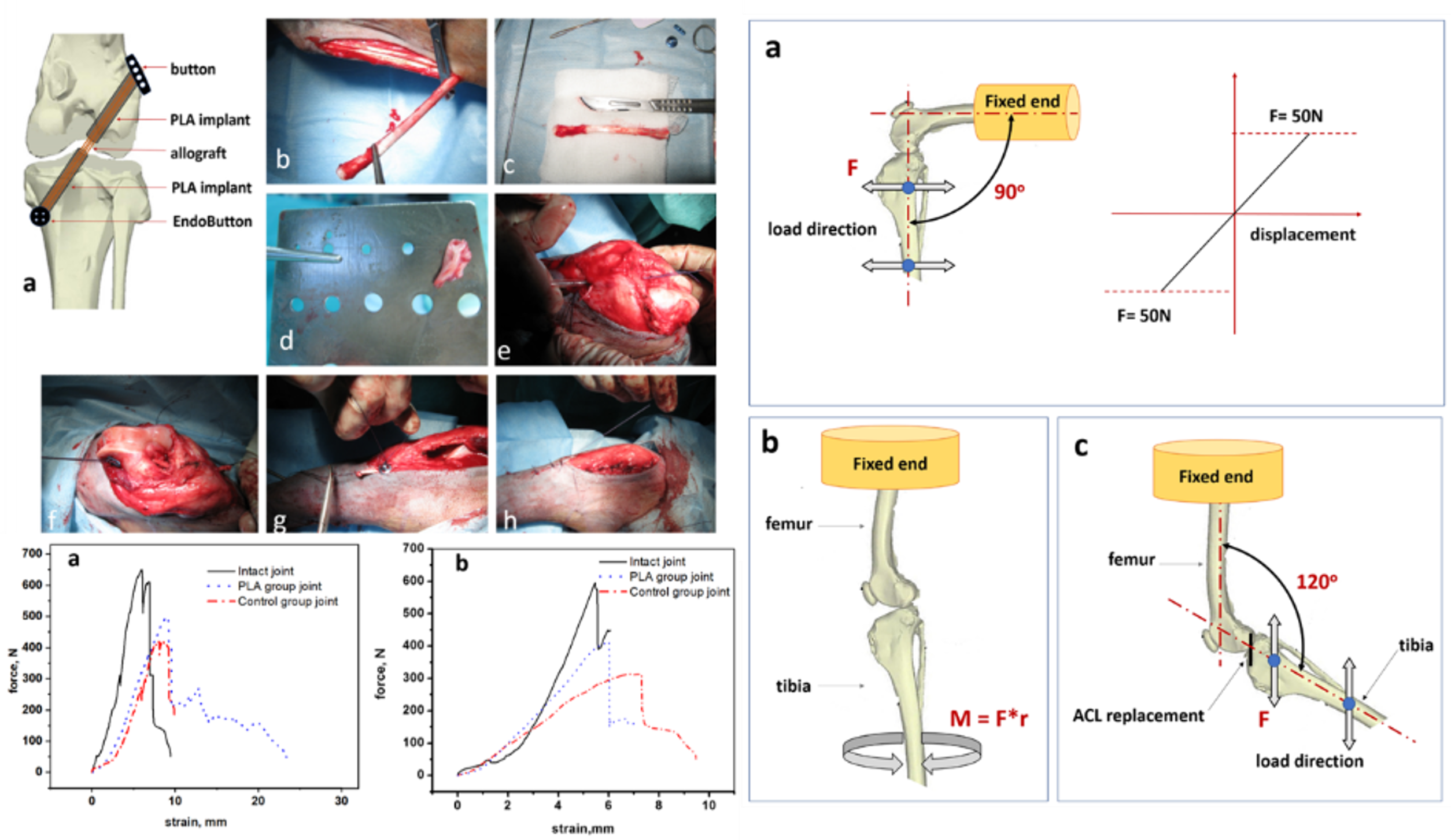
[8]E. Stodolak-Zych, K. Ficek, J. Wieczorek, M. Kajor, K. Gryń, A. Rapacz-Kmita, J. Rajca, Y. Kosenyuk, M. Stolarz, S. Błażewicz Assessment of sheep knee joint after ACL replacement with Achilles tendon autograft and PLA-based implant, DOI: 10.1016/j.jmbbm.2021.104923
Each new material solution is subjected to such procedures in order to verify the behavior in in vivo conditions, assess the effectiveness of the material solution and its surgical convenience. The porous material we developed successfully fills bone defects of various shapes (most often recognized only during the cyst resection in the operating room) due to the proper composition, the microstructure, the form and surgical handiness. Our clinically tested and patented material is an alternative to the golden standard used for treating bone defects [9,10].
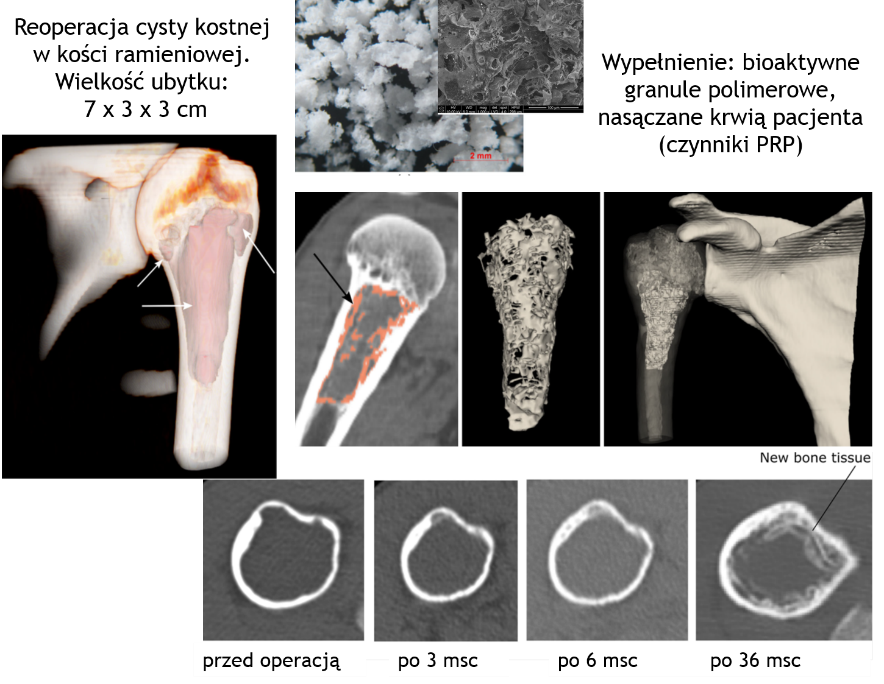
[9]K. Ficek, J. Filipek, P. Wojciechowski, K. Kopeć, E.Stodolak-Zych, S. Błażewicz, A bioresorbable polylactide implant used in bone cyst filling, DOI 10.1007/s10856-015-5647-4
[10] ]E. Stodolak-Zych, A. Magiera, T. Szponder, S. Błazewicz, Sposób wytwarzania resorbowalnej membrany polimerowej dla sterowanej regeneracji tkanek, P.416860 z dn. 1.07.2020
Micrometric carbon fibre biomaterials are used in medicine in the form of nonwovens and oriented fibres (1D, 2D). However, current trends have shifted towards carbon nanomaterials including carbon nanotubes (CNT) and graphene (GR). At the same time, there are many studies reporting controversial nature of both those materials, ie. CNTs. Bearing in mind already proven biocompatibility of amorphous carbon fibres from one side, and vast possibilities of nanoparticles from the other, submicron polyacrylonitrile (PAN) fibres were produced by electrospinning. The polymer was used as a precursor of carbon fibres and, when in the form of a solution, modified with nanoparticles: SiO2 and TCP. Parameters of electrospinning process were optimized for three types of nonwovens: reference PAN nonwoven and nanocomposites: PAN/SiO2 and PAN/TCP. In the next stage, the nonwovens were subjected to two-step thermal conversion. The introduced modifiers enhanced bioactivity of the fibres – apatite-like/Ca-P crystallites layer formed on the surface of the modified nonwovens (simulated body fluid (SBF) assay). The surface of the nanocomposite nonwovens was hydrophilic with a constant value of surface free energy. Finally, biological properties were significantly enhanced: high proliferation rate and better adhesion of osteoblast-like cells (MG-63) were observed (cell number assay, vinculin staining) for the cells cultured on the modified nonwovens as compared to the reference CNF sample.